
Updated Booster Dose Recommendations
On January 5, 2022, the CDC recommended the following updates to COVID-19 booster recommendations:
Everyone 12 years and older in the United States is now recommended to receive a booster dose of the COVID-19 vaccine.
- CDC recommends that children and teens 12 to 17 years and older should receive a Pfizer vaccine booster dose 5 months after completing the second dose of their Pfizer vaccine series. The Pfizer COVID-19 vaccine is the only COVID-19 vaccine currently authorized or approved in the US for people 12 years and older.
- Everyone 18 years and older should receive a booster vaccine. mRNA vaccines (Pfizer and Moderna) are preferred over the J and J vaccine. See Table 1 below Who Can Get a Booster Shot for further details on which vaccine is recommended for your booster dose and how long after your primary series before you are eligible for a booster dose.
The following is attributable to CDC Director, Dr. Rochelle Walensky:
“It is critical that we protect our children and teens from COVID-19 infection and the complications of severe disease. Today, I endorsed ACIP’s vote to expand eligibility and strengthen our recommendations for booster doses. We now recommend that all adolescents aged 12-17 years should receive a booster shot 5 months after their primary series. This boosterdose will provide optimized protection against COVID-19 and the Omicron variant. I encourage
all parents to keep their children up to date with CDC’s COVID-19 vaccine recommendations.”
Is there data to show that boosters work and that they are safe?
There is data to show that COVID-19 boosters help to broaden and strengthen our immune protection against not only Omicron but other COVID-19 virus variants.
CDC’s media statement 12/27/21:
Data from South Africa and the United Kingdom demonstrate that vaccine effectiveness against infection for two doses of an mRNA vaccine is approximately 35%. A COVID-19 vaccine booster dose restores vaccine effectiveness against infection to 75%. COVID-19 vaccination decreases
the risk of severe disease, hospitalization, and death from COVID-19.
Summary recommendations from the ACIP meeting on 1/5/22:
- People who have completed a primary series and booster may be better protected against symptomatic infection with Omicron than those without a booster.
- Two studies from Israel document the effectiveness of Pfizer-BioNTech booster dose 5 months after primary series against severe illness and death secondary to COVID-19.
- 188 million (73%) of U.S. adults aged ≥18 years are fully vaccinated; 38% of those have received a booster.
- 4.74 million (57%) of U.S. adolescents ages 16-17 are fully vaccinated with PfizerBioNTech COVID-19 vaccine; 6% have received a booster.
- Myocarditis cases following mRNA vaccines (Pfizer and Moderna) continue to be followed. These cases are rare. After receiving a booster dose of Pfizer COVID-19 vaccine, 5 months after the primary series, occurrences of myocarditis in people 16 years and older occurred at less than half the rate observed following the 2nd dose of the primary series. CDC scientists continue to monitor this rare occurrence of myocarditis.
Why do we need a booster shot?
- Scientists have discovered that after one gets a COVID-19 vaccine, the protection and immunity they develop to prevent getting COVID-19 infection may decrease over time and also due to the variants that are emerging.
- Although COVID-19 vaccines remain effective in preventing severe disease, recent studies suggest that this effect may decrease over time, particularly in older adults 65 years and older.
- The rapid spread of the Omicron variant further emphasizes the importance of vaccination, boosters, and prevention efforts needed to protect against COVID-19.
- Data from clinical trials showed that a booster shot increased the immune response in trial participants who finished a Pfizer-BioNTech or Moderna primary series 6 months earlier or who received a J&J/Janssen single-dose vaccine 2 months earlier. With an increased immune response, people should have improved protection against getting infected with COVID-19. For Pfizer-BioNTech and J&J/Janssen, clinical trials also showed that a booster shot helped prevent severe disease.
What do we know about the effectiveness and safety of COVID-19 vaccines in children and teens so far?
Members of the ACIP, Advisory Committee on Immunization Practices, met and reviewed data on January 5, 2022, regarding the effectiveness and safety of COVID-19 vaccines
administered to children and teens 5 to 17 years old in the U.S. (primary vaccination series) and 16-24-year-old (booster doses).
Data were reviewed from 3 safety monitoring systems in the United States, VSD, VAERS, and V-safe. The ACIP also reviewed information from Israel and the safety and
effectiveness of Pfizer COVID-19 vaccines administered to children and teens in that country.
A summary of the findings by the ACIP:
- In the setting of the Omicron variant, we are likely to see lower vaccine effectiveness in all populations, compared to vaccine effectiveness seen with the
Delta variant. - Having higher antibody titers improve neutralization of the Omicron variant; booster doses of COVID-19 vaccines do increase neutralization titers.
- Vaccine effectiveness of a booster dose in adolescents 12–15 years of age is still unknown, but a booster dose is likely to provide additional protection (those in this age group were only just authorized to receive a booster dose).
- Of the over 8.7 million doses of Pfizer COVID-19 vaccines administered in the US as of 12/25/21 to children 5-11 years old, and the over 18.7 million doses of Pfizer vaccine administered to 12 to 15-year-olds in the US as of 12/19/21:
- The majority of reported side effects have been mild and resolved within a day or two (including nausea, vomiting, headache, fever, fatigue).
- Children 5-11 reported side effects less often than older children and teens.
- No safety signals were identified among 5-11-year-olds in the VSD (Vaccine Safety Datalink monitoring system).
- Among the 16-24-year-olds who have received a booster dose (over 976,000 doses administered as of 12/19/21) side effects reported were again mild to moderate (including fever, pain at the injection site, chills, headache, nausea fatigue) and mostly occurred a day after vaccination. Side effects were reported less often after the booster shot than after the second dose in the primary series.
Sources:
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-01-05/04-COVIDKlein-508.pdf
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-01-05/02-COVIDSu-508.pdf
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-01-05/03-COVIDHause-508.pdf
Where can I get a booster shot?
Begin with the location that provided or set up your previous COVID-19 vaccine shot. If you need to get your booster shot in a new location, you may utilize the tips included at:
find a vaccine provider, search vaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233 to find locations near you.
What to expect during and after your booster shot appointment:
- Bring your CDC COVID-19 Vaccination Record card to your booster shot appointment so your provider can fill in the information about your booster dose. If you did not receive a card at your first appointment, contact the vaccination site where you got your first shot or your state health department to find out how you can get a card.
- You may experience side effects after getting a COVID-19 vaccine. These are normal signs that your body is building protection against COVID-19.
- Use v-safe to tell CDC about any side effects. If you enter your booster shot in your v-safe account, the system will send you daily health check-ins.
Do booster shots use the same ingredients as existing vaccines?
- Yes, COVID-19 booster shots are the same ingredients (formulation) as the current COVID-19 vaccines. However, the Moderna vaccine booster shot is half the dose of the amount of vaccine people get for their Moderna vaccine primary series. The Pfizer vaccine booster dose is the same dose that is given in the Pfizer vaccine primary series. The J & J vaccine booster dose is the same dose that is given in the primary J & J vaccination.
Table 1. Who can get a booster shot?
Table 2. COVID-19 vaccines: booster dose by primary series
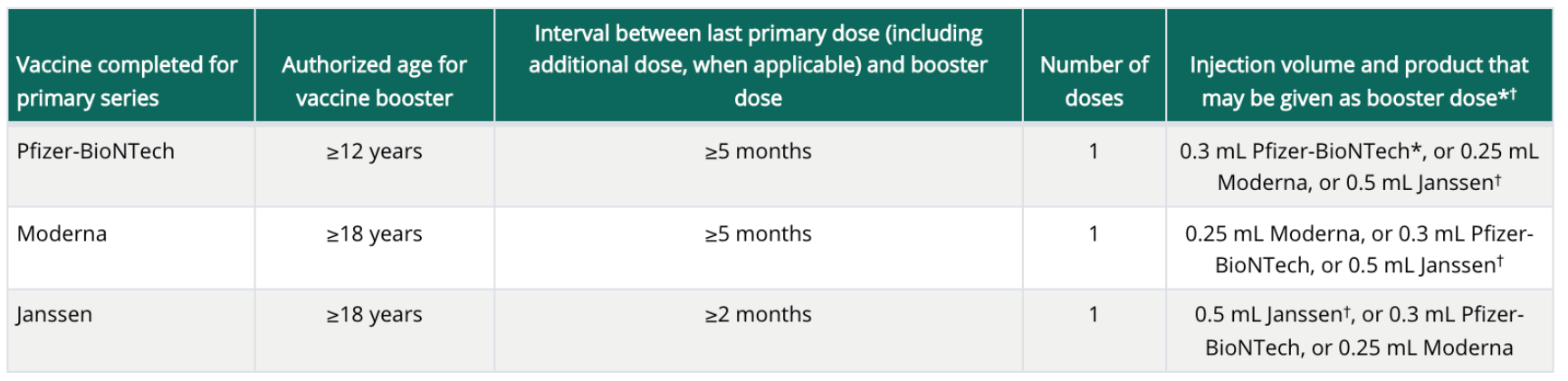
*Only Pfizer BioNTech can be used as a booster dose in thsoe ages 12-17 years.
+Use of an mRNA vaccine for a booster dose is preferred over Janssen vaccine.
*Although mRNA vaccines are preferred, the J&J/Janssen COVID-19 vaccine may be considered in some situations.
Other resources: https://publichealthcollaborative.org/faq/#Vaccine-Booster-Doses
Updated Recommendations for COVID-19 Vaccination in
Immunocompromised Persons
CDC and FDA scientists now recommend that some children ages 5 to 11 years old who are immunocompromised should receive an additional dose of Pfizer BioNTech COVID-19 vaccine as part of their primary vaccination series.
- People with immunocompromising conditions or people who take immunosuppressive medications or therapies are at increased risk for severe COVID-19.
- Vaccine effectiveness is lower among patients who are immunocompromised and furthermore, their protection by primary vaccination may wane over time making them susceptible to severe SARS-CoV-2 infection (COVID-19 infection).
- CDC now recommends an additional primary series mRNA vaccine dose in immunocompromised people 5 years and older. This is consistent with the prior similar recommendation for immunocompromised adults.
- CDC is recommending that moderately or severely immunocompromised 5 to 11-year-olds receive an additional primary dose of vaccine 28 days after their second shot. At this time, only the Pfizer-BioNTech COVID-19 vaccine is authorized and recommended for children aged 5-11. Children 5-11 years old who are moderately or severely immunocompromised will now receive 3 doses of Pfizer COVID-19 vaccine in the primary series.
Source: https://www.cdc.gov/media/releases/2022/s0104-Pfizer-Booster.html
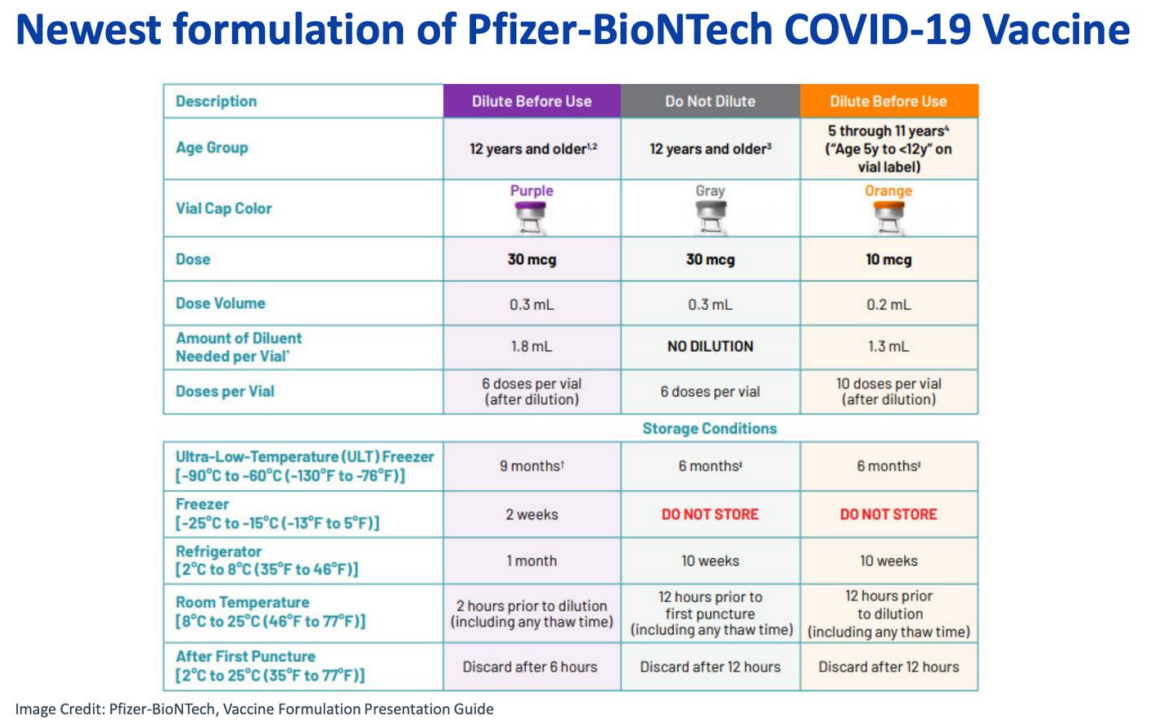
New Formulation of Pfizer-BioNTech COVID-19 Vaccine (Gray Top) for
People 12 Years and Older
Second formulation of Pfizer-BioNTech COVID-19 Vaccine (Gray Top) for people 12 years and older
There is now a second formulation of the Pfizer-BioNTech COVID-19 vaccine for ages 12 years and older which does not require dilution. It also does not require freezing. It is a gray-top vial. For details on this new formulation, please refer to the table below.
Quarantine/Isolation Recommendations
On January 4, 2022, CDC updated its COVID-19 isolation and quarantine recommendations with shorter isolation (for asymptomatic and mildly ill people) and quarantine periods of 5 days to focus on the period when a person is most infectious, followed by continued masking for an additional 5 days
Why did CDC update its recommendations on quarantine and isolation?
CDC has continued to review isolation and quarantine recommendations for various populations throughout the COVID-19 pandemic. Accumulating evidence demonstrates that the majority of transmission occurs during the early periods of infection. These updated recommendations come as the Omicron variant is now rapidly spreading throughout the United States causing very high case rates. The recommendations reflect the societal impact (e.g., critical infrastructure and staffing shortages) and the latest science on disease severity and when and for how long a person is maximally infectious.
Quarantine and Isolation
You quarantine when you might have been exposed to the virus and may or may not have been infected. You isolate when you are sick or when you have been infected with the virus, even if you don’t have symptoms.
Who should quarantine?
If you come into close contact with someone with COVID-19, you should quarantine if you are in one of the following groups:
- You are ages 18 or older and completed the primary series of recommended vaccine, but have not received a recommended booster shot when eligible.
- You received the single-dose Johnson & Johnson vaccine (completing the primary series) over 2 months ago and have not received a recommended booster shot.
- You are not vaccinated or have not completed a primary vaccine series.
What to do for quarantine
- Stay home and away from other people for at least 5 days after your last contact with a person who has COVID-19. Wear a well-fitting mask when around others at home, if possible.
- For 10 days after your last close contact with someone with COVID-19, watch for fever (100.4◦F or greater), cough, shortness of breath, or other COVID-19 symptoms.
- If you develop symptoms, get tested immediately and isolate until you receive your test results. If you test positive, follow isolation recommendations.
- If you do not develop symptoms, get tested at least 5 days after you last had close contact with someone with COVID-19.
- If you test negative, you can leave your home, but continue to wear a well fitting mask when around others at home and in public until 10 days after your last close contact with someone with COVID-19.
- If you test positive, you should isolate for at least 5 days from the date of your positive test (if you do not have symptoms). If you do develop COVID-19 symptoms, isolate for at least 5 days from the date your symptoms began (the date the symptoms started is day 0). Follow recommendations in the isolation section below.
- If you are unable to get a test 5 days after last close contact with someone with COVID-19, you can leave your home after day 5 if you have been without COVID-19 symptoms throughout the 5-day period. Wear a well fitting mask for 10 days after your date of last close contact when around others at home and in public.
- Avoid people who are immunocompromised or at high risk for severe disease, nursing homes, and other high-risk settings until after at least 10 days.
- If possible, stay away from people you live with, especially people who are at higher risk for getting very sick from COVID-19, as well as others outside your home throughout the full 10 days after your last close contact with someone with COVID-19.
- If you are unable to quarantine, you should wear a well-fitting mask for 10 days when around others at home and in public.
- If you are unable to wear a mask when around others, you should continue to quarantine for 10 days. Avoid people who are immunocompromised or at high risk for severe disease, nursing homes, and other high-risk settings until after at least 10 days.
- Do not travel during your 5-day quarantine period. Get tested at least 5 days after your last close contact and make sure your test result is negative and you remain without symptoms before traveling. If you don’t get tested, delay travel until 10 days after your last close contact with a person with COVID-19. If you must travel before the 10 days are completed, wear a well-fitting mask when you are around others for the entire duration of travel during the 10 days. If you are unable to wear a mask, you should not travel during the 10 days.
- Do not go to places where you are unable to wear a mask, such as restaurants and some gyms, and avoid eating around others at home and at work until after 10 days after your last close contact with someone with COVID-19.
After quarantine, watch for symptoms until 10 days after your last close contact with someone with COVID-19. If you have symptoms, isolate immediately and get tested. See CDC guidance on quarantine in high-risk congregate settings.
Who does not need to quarantine?
If you came into close contact with someone with COVID-19 and you are in one of the following groups, you do not need to quarantine.
- You are ages 18 or older and have received all recommended vaccine doses, including boosters and additional primary shots for some immunocompromised people.
- You are ages 5-17 years and completed the primary series of COVID-19 vaccines.
- You had confirmed COVID-19 within the last 90 days (you tested positive using a viral test).
You should wear a well-fitting mask around others for 10 days from the date of your last close contact with someone with COVID-19. Get tested at least 5 days after you last had close contact with someone with COVID-19. If you test positive or develop COVID-19 symptoms, isolate from other people and follow the isolation recommendations. If you tested positive for COVID-19 with a viral test within the previous 90 days and have since recovered and remain without COVID-19 symptoms, you do not need to quarantine or get tested after close contact. You should wear a well-fitting mask around others for 10
days from the date of your last close contact with someone with COVID-19.
Who needs to isolate?
- People who have a positive viral test for COVID-19, regardless of whether or not they have symptoms.
- People with symptoms of COVID-19, including people who are awaiting test results or have not been tested. People with symptoms should isolate even if they do not know if they have been in close contact with someone with COVID-19.
What to do for isolation
- Monitor your symptoms. If you have an emergency warning sign (including trouble breathing), seek emergency medical care immediately.
- Stay in a separate room from other household members and use a separate
bathroom, if possible. - Take steps to improve ventilation at home, if possible.
- Avoid contact with other members of the household and pets.
- Don’t share personal household items, like cups, towels, and utensils.
- Wear a well-fitting mask when you need to be around other people.
Ending isolation
Isolate for at least 5 days. To calculate your 5-day isolation period, day 0 is your first day of symptoms. Day 1 is the first full day after your symptoms developed. You can leave isolation after 5 full days.
- For those who had symptoms, you can end isolation after 5 full days if you are fever-free for 24 hours without the use of fever-reducing medication and your other symptoms have improved (loss of taste and smell may persist for weeks or months after recovery and need not delay the end of isolation). If you continue to have fever or your other symptoms have not improved after 5 days of isolation, you should wait to end your isolation until you are fever-free for 24 hours without the use of fever-reducing medication and your other symptoms have improved. Continue to wear a well-fitting mask. Contact your healthcare provider if you have questions.
- For those who had no symptoms, if you continue to have no symptoms, you can end isolation after at least 5 days. If you develop symptoms after testing positive, your 5-day isolation period should start over. Day 0 is your first day of symptoms. Follow the recommendations above for ending isolation for people who had COVID-19 and had symptoms.
- You should continue to wear a well-fitting mask around others at home and in public for 5 additional days (day 6 through day 10) after the end of your 5-day isolation period. If you are unable to wear a mask when around others, you should continue to isolate for a full 10 days. Avoid people who are immunocompromised or at high risk for severe disease, nursing homes, and other high-risk settings until after at least 10 days.
- If an individual has access to a test and wants to test, the best approach is to use an antigen test (1) towards the end of the 5-day isolation period. Collect the test sample only if you are fever-free for 24 hours without the use of fever-reducing medication and your other symptoms have improved (loss of taste and smell may persist for weeks or months after recovery and need not delay the end of isolation). If your test result is positive, you should continue to isolate until day 10. If your test result is negative, you can end isolation, but continue to wear a well-fitting mask around others at home and in public until day 10. Follow additional recommendations for masking and restricting travel as described above.
1 – Negative results should be treated as presumptive. Negative results do not rule out SARSCoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. To improve results, antigen tests should be used twice over a three-day period with at least 24 hours and no more than 48 hours between tests.
Note that these recommendations on ending isolation do not apply to people with severe COVID-19 or with weakened immune systems (immunocompromised). See CDC recommendations for immunocompromised people.
Social Media Posts
Talking points, like those above, are meant for just that — talking. They shouldn’t be used verbatim in print, email or social media.
Talking points are most effective when you use your own language to share the basic information found in said content, sharing messages in a style of speech that is both expected and best understood by your audiences. For social media, that means keeping it short, conversational and not trying to tackle too much information at once. Stick to the most important details, and don’t try to explain too much in a single post.
You wouldn’t read Shakespeare to a fifth-grade class; instead, you would talk about the general themes of Shakespeare’s stories and avoid the complicated language. We suggest a similar approach to using critical vaccine information on social media.
For example:
Twitter (limited to 280 characters with spaces):
CDC recommends everyone 12-17 years receive a Pfizer COVID-19 vaccine
#boosterdose 5 months after completing their second dose. https://www.cdc.gov/media/releases/2022/s0105-Booster-Shot.html
All adults are also eligible for a vaccine #booster. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
Avoid #Flurona – get your flu and COVID-19 vaccines!
Everyone 12 years and older should receive a COVID-19 #boosterdose when eligible!
Avoid Flurona – get your flu and COVID-19 vaccines! Protect your friends and family by getting vaccinated against COVID-19 and the flu. Everyone 5 years and older is eligible for COVID-19 vaccine and 6 months and older for flu vaccine.
CDC recommends everyone 12-17 years receive a Pfizer COVID-19 vaccine booster dose 5 months after completing their second dose.
All adults are also eligible for a vaccine booster. Details at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
