Download the Monoclonal Antibodies for COVID-19 Disease Talking Points Document
This talking points document was developed by iREACH Subject Matter Experts Dr. Yabo Beysolow and Lisa Jacques-Carroll to provide information on monoclonal antibodies: what they are, how they work, and how they should be used.
Monoclonal Antibodies: What they are, how they work and how they should be used
- As of September 16, 2021, there are three COVID-19 monoclonal antibodies authorized by the FDA currently under an EUA. They are authorized to be given to patients who are NOT hospitalized but who test positive for COVID-19 virus and are at high risk for progressing to more serious disease or hospitalization (e.g., those with high blood pressure, heart disease, a body mass index (BMI) higher than 30, an autoimmune disorder, people taking immunosuppressant drugs, and people who are pregnant).
- COVID-19 monoclonal antibodies are effective in preventing worsening or severe COVID-19 disease, hospitalization or death due to COVID-19 disease in someone who has tested positive to COVID-19 virus and have mild to moderate symptoms.
- COVID-19 monoclonal antibodies have to be prescribed by a health care provider. All treatments used for COVID-19 should be prescribed by your healthcare provider. People have been seriously harmed and even died after taking products not approved for COVID-19, even products approved or prescribed for other uses.
- COVID-19 monoclonal antibodies help to prevent someone who is not in the hospital but has mild or moderate COVID-19 disease (tested positive for COVID-19) and are at high risk of getting worse, from getting severely ill from COVID-19 disease and ending up in the hospital.
- COVID-19 monoclonal antibodies work by giving your body antibodies (fighting cells) to fight off the spike protein (antigen) from the Covid-19 virus. The monoclonal antibodies attach to the spike protein of the virus. They allow our body’s immune system to recognize and respond better to the virus.
- Some COVID-19 monoclonal antibodies may not work as well if someone is already severely ill, in the ICU or on a ventilator.
- The FDA’s EUA does not currently authorize the use of monoclonal antibodies in patients who are hospitalized due to COVID-19 disease, or those who require oxygen therapy due to COVID-19 disease.
- There are exceptions to this, and a health care provider will make decisions about the appropriate use of monoclonal antibodies.
- COVID-19 monoclonal antibodies are administered in a health care setting under the guidance of a licensed health care provider either as a series of injections or through an IV treatment.
- Side effects may occur including, rash, diarrhea, nausea, dizziness, itching or severe allergic reactions.
- COVID-19 monoclonal antibodies work best when they are given to someone with a positive test as soon as possible or within 7 to 10 days of when they develop any symptoms of COVID-19.
- Some COVID-19 monoclonal antibodies may be free to patients, through funding by the Federal Government. To ensure adequate supplies for all States, HHS determines the amount of product each state and territory receives on a weekly basis to ensure equitable distribution across the country.
- The goal of giving someone COVID-19 monoclonal antibody therapy is to directly treat someone infected with COVID-19 virus and prevent worsening infection (in high-risk settings). Monoclonal antibody therapy is used to treat infections that have already occurred.
- Your healthcare provider will decide whether COVID-19 monoclonal antibodies are appropriate to treat your illness. You will need a doctor’s prescription to receive monoclonal antibodies.
- Some monoclonal antibodies may not work as well against variants. The FDA continues to monitor all authorized monoclonal antibodies to see if they will work against COVID-19 variants, including the Delta variant.
- For people with breakthrough infections (meaning developing COVID-19 disease after having had the COVID-19 disease), monoclonal antibodies may be considered by the healthcare provider.
- If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine to avoid the antibody treatment interfering with how well the vaccine may work in you.
Additional Use of Monoclonal Antibodies
- Post-exposure prophylaxis is when a medication/treatment is given to someone who has been exposed to an illness, in order to help prevent them from coming down with that illness.
- One of the COVID-19 monoclonal antibodies authorized by the FDA, REGEN-COV, may be given as POST-exposure prophylaxis in persons who at high risk for severe COVID-19 disease, hospitalization or death, who are:
- Not fully vaccinated or not expected to have a good immune response to vaccination because they are immunocompromised (from a condition or medication).
- Have been exposed to a close contact who is infected with COVID-19 virus or who are at high risk of exposure to a close contact infected with COVID-19 virus (for example in a nursing home or prison).
- Monoclonal antibodies are NOT authorized to be used as PRE-exposure prophylaxis to prevent COVID-19 disease. In other words, these treatments are not authorized to be used before an exposure to a person/people known to have COVID-19 infection in order to avoid the disease.
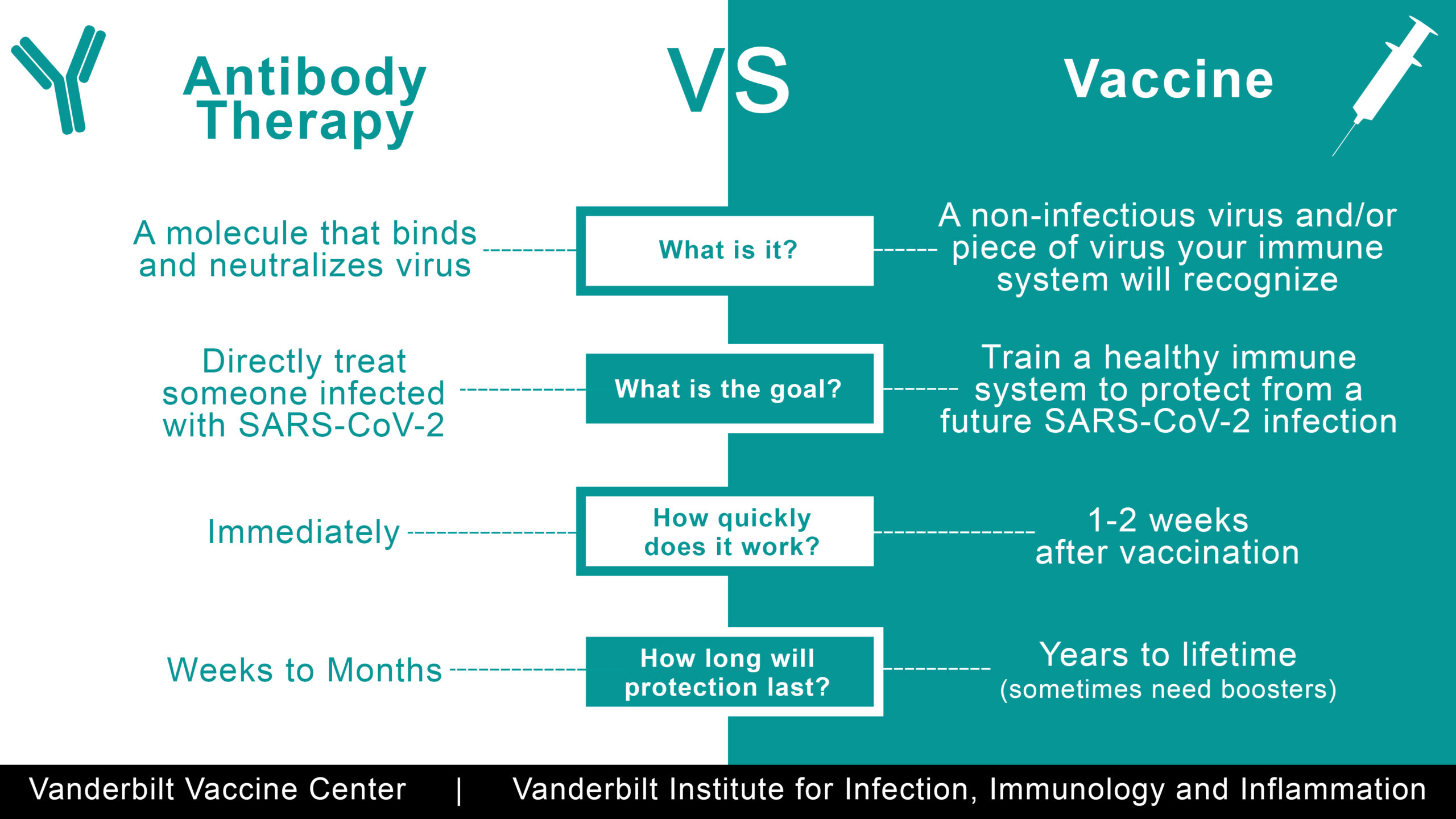
Differences between monoclonal antibodies and COVID-19 vaccines
- Monoclonal antibodies are NOT vaccines, they are a treatment. They are given to treat someone with COVID-19 infection.
- Monoclonal antibodies are NOT a substitute for COVID-19 vaccines.
- COVID-19 monoclonal antibodies work immediately when given to a patient, however the protection from monoclonal antibodies wears off faster than the protection from a vaccine. Some scientists believe protection may last up to a month or longer. Scientists are still learning the duration of protection.
- COVID-19 monoclonal antibodies work by giving your body antibodies (fighting cells) to fight off the spike protein (antigen) from the Covid-19 virus. The monoclonal antibodies attach to the spike protein of the virus. They allow our body’s immune system to recognize and respond better to the virus.
- Vaccines are given to prevent infection. It takes up to 2 weeks after getting a COVID-19 vaccine to begin receiving protection.
- The goal of giving someone a COVID-19 vaccine is to train a healthy immune system to protect from a future COVID-19 Infection by developing antibodies (fighting cell against the spike protein). For several months after COVID-19 vaccination, your body continues to produce these fighting cells. Protection from vaccines may last months to years.
- When a COVID-19 vaccine is injected into a person, their immune system responds as if the person were infected with SARS-CoV-2. However, the person will not become sick with COVID-19 disease. Instead, the person develops what is known as “immune memory.” Since the body has already learnt how to adapt to the virus, immune memory protects it from infection in the future, helping the body to clear the COVID-19 virus before it can do any damage. This protective immunity may last for months, years or even a lifetime in certain cases.
- If your body is exposed to the COVID-19 virus after you have been vaccinated and have developed antibodies, your body is ready to fight off the infection and avoid getting ill. Breakthrough infections are rare and usually lead to a mild infection. COVID-19 vaccination protects you from being hospitalized or dying in most cases, even if you get a breakthrough infection.
- With rare exceptions, persons 12 years and older do not need a doctor’s prescription to get a COVID-19 vaccine.
- Vaccines will not treat you or cure you if you already have COVID-19 disease.
Additional treatments for COVID-19 disease for hospitalized patients
- Antiviral medications can be used to reduce the ability of the virus to multiply and spread through the body.
- In patients with severe COVID-19, the body’s immune system may overreact to the threat of the virus, worsening the disease. This can cause damage to the body’s organs and tissues. Some treatments can help reduce this overactive immune response.
- COVID-19 can damage the heart, blood vessels, kidneys, brain, skin, eyes, and gastrointestinal organs. It also can cause other complications. Depending on the complications, additional treatments might be used for severely ill hospitalized patients, such as blood thinners to prevent or treat blood clots.
- Plasma from patients who have recovered from COVID-19—called convalescent plasma—can contain antibodies to the virus. This could help the immune system recognize and respond more effectively to the virus, but currently the NIH find that there is not enough evidence to recommend these treatments.
Social Media Posts
Talking points, like those above, are meant for just that — talking. They shouldn’t be used verbatim in print, email or social media.
Talking points are most effective when you use your own language to share the basic information found in said content, sharing messages in a style of speech that is both expected and best understood by your audiences. For social media, that means keeping it short, conversational and not trying to tackle too much information at once. Stick to the most important details, and don’t try to explain too much in a single post.
You wouldn’t read Shakespeare to a fifth-grade class; instead, you would talk about the general themes of Shakespeare’s stories and avoid the complicated language. We suggest a similar approach to using critical vaccine information on social media.
Twitter (280 character limit)
If you or someone you know is sick with #COVID19 you may qualify for monoclonal antibodies to prevent severe illness and keep you out of the hospital.
Monoclonal antibodies can help people sick with #COVID19, but #COVID19vaccines provide the best protection against becoming infected with COVID-19.
If you or someone you know is sick with COVID-19 you may qualify for monoclonal antibodies to prevent severe illness and keep you out of the hospital. Talk to your doctor to see if you are a candidate for monoclonal antibodies.
Monoclonal antibodies can help people sick with COVID-19, but COVID-19 vaccines provide the best protection against becoming infected with COVID-19. Monoclonal antibodies are meant to be used as treatment for COVID-19 infection to prevent severe illness.
Additional Resources
- REACH resource: COVID-19 Treatments
- Infographics from Vanderbilt Institute for Infection, Immunology and Inflammation
- FDA Know your treatment options for COVID-19
- HHS Combat COVID
- NIH COVID-19 Treatment Guidelines with monoclonal antibodies
- NIH COVID-19 Treatment guidelines with convalescent plasma
- CDC: Interim Clinical Considerations for use of COVID-19 vaccines
- CDC: Treatments your healthcare provider may recommend if you are sick with COVID-19 disease
- CDC COCA Call (webinar recording and slides) Therapeutic options to prevent severe COVID-19 in immunocompromised people
- HHS.GOV resources for Health care providers
This resource is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) as part of a Cooperative Agreement. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.