This toolkit is designed to equip members of the Association of Immunization Managers (AIM) and their staff with the tools and information necessary to appropriately and effectively engage with elected officials. You’ll find a selection of immunization program activities and strategies for appropriately and effectively promoting sound immunization policies.
Immunization Program Policy Resource Guide
Legislators at the federal and state levels have enormous influence on policies and programs administered by state and local immunization program (IP) managers. By engaging with elected officials, IP managers support program efforts to rid the nation of vaccine-preventable diseases, ensure adequate resources for programs, and promote sound immunization policies.
Public health officials are often surprised to learn that many elected officials and their staff want to hear from immunization programs. Program managers and staff are the experts on immunization programs and can uniquely demonstrate the extraordinary value of the scarce public resources allocated to immunization programs.
Program managers are the best advocates for what the programs they lead need to be effective. If policymakers don’t hear from immunization programs, it’s possible they may only hear from those who are opposed to what they do.
That’s why it’s imperative for immunization program managers to engage in the policy process in an ethically responsible manner. The three chapters of this resource guide provide insight into how to do this. You can download a PDF of the whole guide, or each individual chapter.
Immunization Program Policy Resource Guide: Chapter 1
Chapter 1: Advocacy Rules and the Legislative Process

Legislators at the federal and state levels have enormous influence on policies and programs administered by state and local immunization program managers. By engaging with elected officials, immunization program managers support program efforts to rid the nation of vaccine-preventable diseases, ensure adequate resources for programs, and promote sound immunization policies.
Chapter 1 of the Immunization Program Policy Guide covers the basics of program policy, including how to distinguish among education, advocacy, and lobbying, an overview of the relevant rules and regulations to ensure appropriate engagement, and fundamental information about the legislative process to guide effective action.
Immunization Program Policy Resource Guide: Chapter 2
Chapter 2: The Public Policy Landscape and Key Partners

To maximize your effectiveness as a proponent for sound immunization policy, it helps to make periodic assessments of the policy landscape in your state or jurisdiction. Understanding key players’ roles and building relationships with them will allow you to anticipate, as well as shape, agendas where appropriate.
Chapter 2 of the Immunization Program Policy Resource Guide discusses the key players that shape the immunization policy landscape in your state, highlights the benefits of partnering with these key players, and offers suggestions for building effective relationships with them.
Immunization Program Policy Resource Guide: Chapter 3
Chapter 3: Effective Strategies for Educating Policymakers

Effective education utilizes a range of tools to shape public debate and inform sound policy. Chapter 3 of the Immunization Program Policy Guide highlights several effective approaches to educating the public and policymakers, including developing outreach plans, building personal relationships, and working with the media to deliver compelling messages. Engaging coalition partners, anticipating opposition, and planning to evaluate are also covered.
Immunization program managers share key insights, lessons learned and steps for appropriately and effectively promoting sound immunization policies.
Preparing for the 2021 Legislative Session
On January 14, 2021, this webinar provided a legislative update, tips for managing the 2021 legislative session, and a background on mandates.
Advocacy Rules and the Legislative Process Webinar
On September 17, 2019 the webinar covered the difference between education, advocacy, and lobbying, relevant rules and regulations, and fundamentals of the legislative process. Program managers Ron Balajadia (HI) and Tonya Philbrick (ME) joined the webinar to share their perspectives.
Mandates
National Academy for State Health Partnership Map
Visit NASHP’s interactive map of states that are taking action on COVID-19 vaccine mandates and passports.
State/Federal Authority to Mandate COVID Vaccine
Report from the Congressional Research Service on State and Federal Authority to Mandate COVID-19 Vaccination
Daycare & School Requirements
Back to School/Catch-Up Immunization Webinar
AIM hosted a Back to School/Catch-Up Immunization webinar with partners from CDC, Unity Consortium, National Association of School Nurses (NASN),…
ND Testimony on House Bill 1320
Testimony from the ND IP on the importance of childcare, school, and university immunization requirements.
Exemptions
National Academy for State Health Partnership Map
Visit NASHP’s interactive map of states that are taking action on COVID-19 vaccine mandates and passports.
CT Legislator Questions about Exemptions
On February 19, 2020, the Connecticut General Assembly’s Public Health Committee held a hearing on House Bill 5044, which would…
General COVID-19
National Academy for State Health Partnership Map
Visit NASHP’s interactive map of states that are taking action on COVID-19 vaccine mandates and passports.
Tips: Working Together to Address Misinformation
This tip sheet was developed by AIM and NPHIC to help immunization program managers and public information officers collaborate effectively.
Parental/Minor Consent
AMA Model Legislation for “Mature Minor” Consent
AMA discussion of implications of immunization policy regarding patient, parent, and physician relationships and informed consent. Furthermore, this document discusses…
Informed Consent/Education
Rhode Island House Bill 6015
The bill would require immunization informed consent forms to include information on the vaccine’s manufacturing details, potential adverse reactions, vaccine…
OR Testimony on Senate Bill 869
2017 Testimony from OR IP on Senate Bill 869, which requires providers to distribute the VIS, explain vaccine risks and…
Immunization Information Systems
AIM Policy Maps
AIM policy maps are updated on a rolling basis with voluntary information that is self-reported by immunization programs.
Communicating the Value of Immunization Information Systems (IIS): A Toolkit for Program Managers
The IIS Communications Toolkit includes sample language, templates, slides, and talking points to communicate the value and importance of IIS to public health.
Pharmacy
Oregon: Evaluating the Impact of a New Pharmacist Vaccination Law
The Oregon Immunization Program evaluated the impact of a change in Oregon pharmacy law on adolescent influenza vaccination.
Nevada: Enrolling Pharmacies in the VFC Program
The Nevada Department of Health and Human Service Immunization Program enrolled pharmacies in the VFC program in response to new…
Vaccine Ingredients
HHS Fact Sheet on Vaccine Ingredients
Vaccine ingredient facts from a federal government Website managed by the Office of Infectious Disease and HIV/AIDS Policy, U.S. Department…
ND Vaccine Facts for the Legislature
This document was created by the North Dakota Immunization Program for legislators with facts about vaccines. It covers a wide…
Safety & Effectiveness
Aluminum Adjuvants Talking Points
Vaccines include ingredients to help them do their work. Each ingredient used to make a vaccine has a specific purpose. Certain ingredients, just like preservatives in foods, help keep vaccines safe from contamination and toxins that could make us sick.
COVID-19 Vaccines: From the Lab to Your Arm
This iREACH infographic illustrates the process of how vaccines are developed, giving insight into the entire vaccine process.
Investments in Immunization
Summary: The Value of Vaccines Report
Notes from AIM staff on the Value of Vaccines report which summarizes recent evidence demonstrating how vaccines provide incredible value…
ROI Brief – The Value of Vaccine Programs
Digestible brief from Johns Hopkins Bloomberg School of Public Health and the International Vaccine Access Center on new return on…
Other
2024/2025 State Legislative Sessions Report
This report is a comprehensive review of all proposed, considered, enacted, and vetoed vaccine-related legislation that AIM monitored over the past year.
2023/2024 State Legislative Sessions Report
This report is a comprehensive review of all proposed, considered, enacted, and vetoed vaccine-related legislation that AIM monitored over the past year.
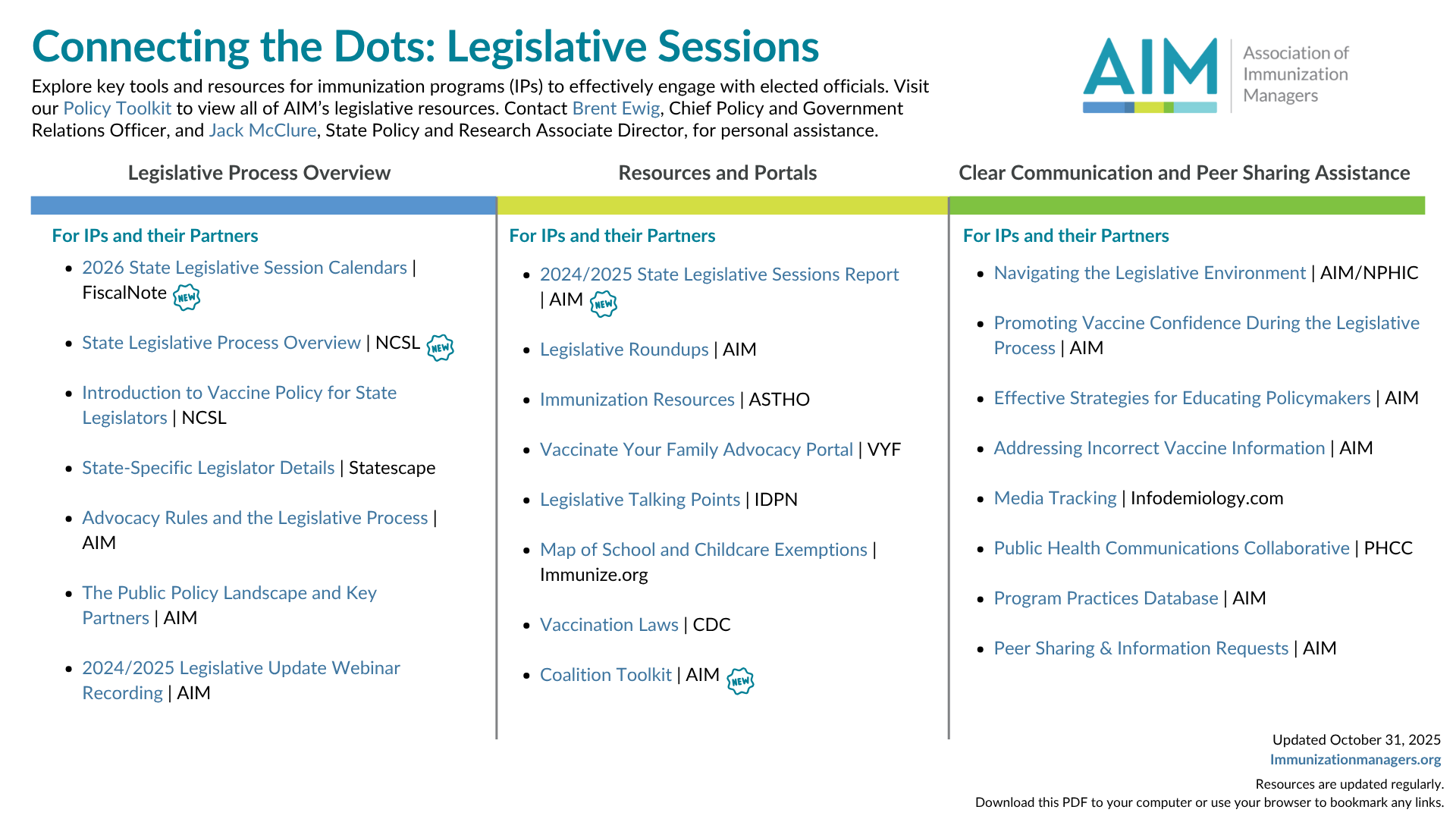
Connecting the Dots: Legislative Sessions
AIM’s Connecting the Dots: Legislative Sessions is a regularly updated resource that provides a centralized location to find resources pertaining to state legislative systems, including legislative process overview, resources and portals, clear communication guidelines, and peer sharing and assistance. Please contact Brent Ewig for legislative questions and Rachel Van Gundy for any other questions or concerns.
Legislative Round-Up
AIM’s Legislative Round-Ups are bi-weekly recaps on immunization-related legislation.
AIM Legislative Round-up: May 30, 2025
Vaccine-related legislated movement has slowed significantly as most state sessions come to an end. This is the last bi-weekly Legislative Round-up of 2025.
AIM Legislative Round-up: May 16, 2025
Half of the state’s legislative sessions have concluded for the year, with 25 states still underway.
AIM Legislative Round-up: May 2, 2025
Legislative movement has slowed down as many state legislatures near adjournment, but several of the 538 vaccine-related bills AIM is tracking have faced votes.
Policy Summary
AIM’s Policy Summaries are weekly recaps on legislation being introduced, in action, and that has passed.
AIM Policy Summary: August 5, 2022
Activity has slowed as remaining legislative sessions come to a close. Through the summer, AIM staff will compile new or moving legislation in jurisdictions.
AIM Policy Summary: July 22, 2022
Despite season lateness, legislatures are enacting immunization-related legislation. Read up on the latest legislation in jurisdictions compiled by AIM staff.
AIM Policy Summary: July 1, 2022
Read up on the latest news around fast-moving legislation in jurisdictions around the US compiled by the AIM staff.
Legislative Tips in Two
Two Minute Tips are for members only. If you haven’t logged in, please log in to view.
These three infographics were designed to promote AIM’s advocacy priorities, as voted on by the AIM Executive Committee. Immunization leaders are encouraged to use them as educational references when speaking to vaccine partners, interested parties, and policy makers.
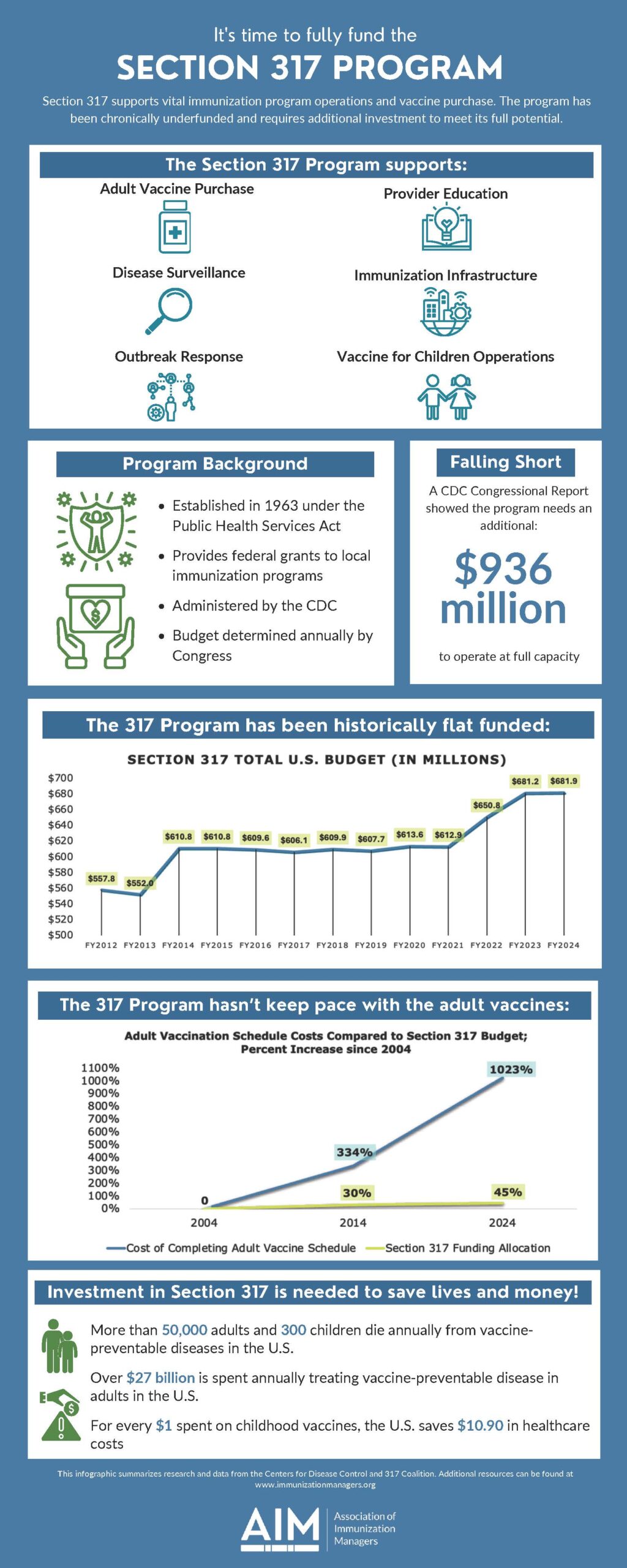
Section 317 Program
This infographic on Section 317 was designed to promote AIM’s advocacy priorities, as voted on by the AIM Executive Committee. Immunization leaders are encouraged to use it as an educational reference when speaking to vaccine partners, interested parties, and policy makers.
Section 317 supports vital immunization program operations and vaccine purchase. The program has been chronically underfunded and requires additional investment to meet its full potential.
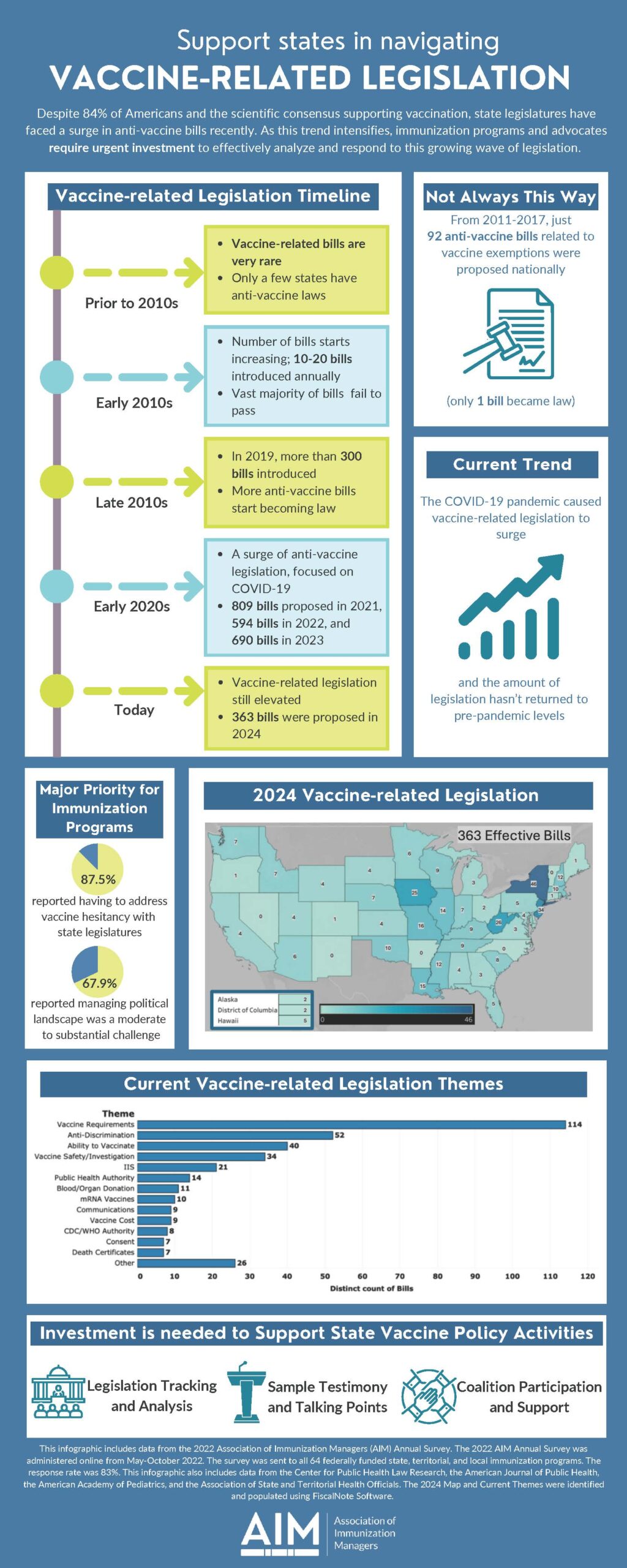
Vaccine-Related Legislation
This infographic on navigating vaccine-related state legislation was designed to promote AIM’s advocacy priorities, as voted on by the AIM Executive Committee. Immunization leaders are encouraged to use it as an educational reference when speaking to vaccine partners, interested parties, and policy makers.
Despite 84% of Americans and the scientific consensus supporting vaccination, state legislatures have faced a surge in anti-vaccine bills recently. As this trend intensifies, immunization programs and advocates require urgent investment to effectively analyze and respond to this growing wave of legislation.
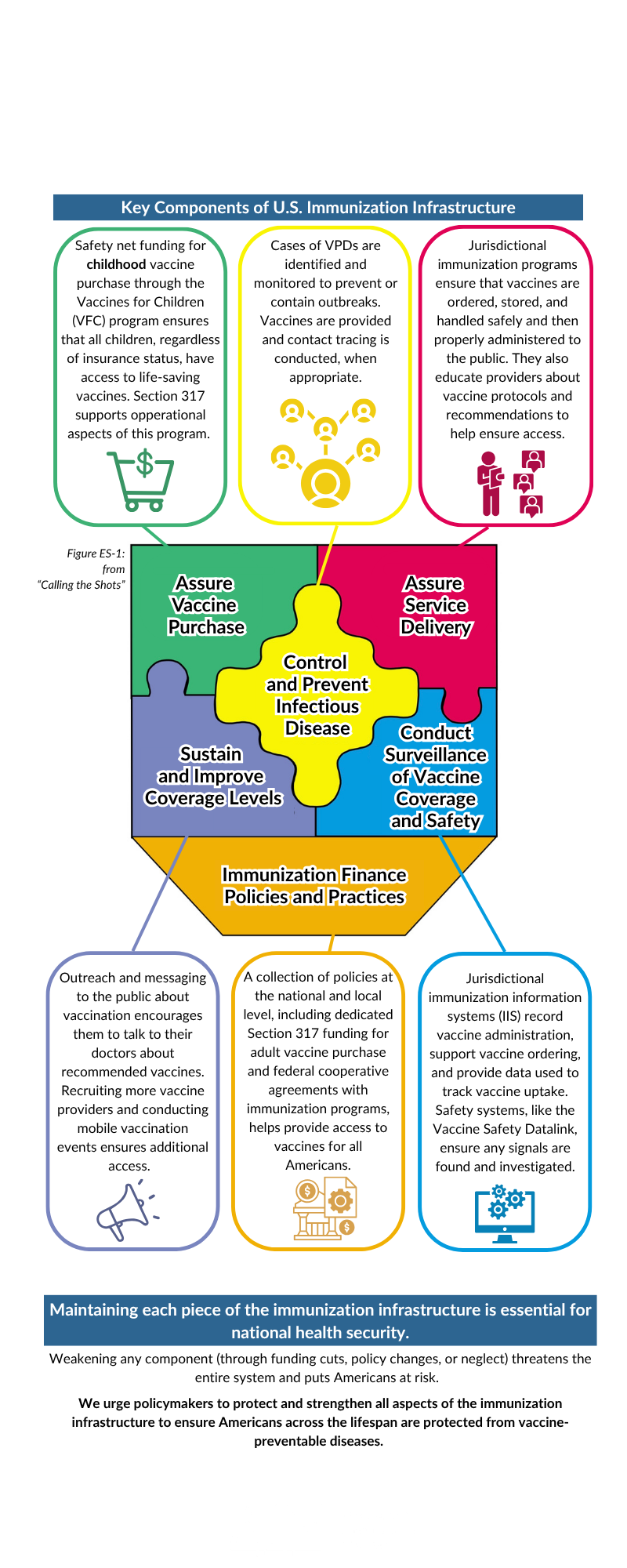
Immunization Infrastructure
This infographic on immunization infrastructure was designed to promote AIM’s advocacy priorities, as voted on by the AIM Executive Committee. Immunization leaders are encouraged to use it as an educational reference when speaking to vaccine partners, interested parties, and policymakers.
The nation’s protection against vaccine-preventable diseases (VPDs) depends on complex, interconnected components that together comprise our nation’s vaccine infrastructure. Each piece is vital—remove or weaken one, and the whole system is at risk.
For More Information
Need help with activities related to upcoming legislation? Contact AIM Chief Policy and Government Relations Officer Brent Ewig.